Multifunctional pharma grade excipients for dry oral forms
Alsiano supplies a broad range of MCC microcrystalline cellulose with very low levels of nitrite and nitrate offering special functionalities for pharmaceutical applications.
Further to MCC, we offer the pharma grade excipient SEPISTAB ST 200 PHARMA with binding and disintegrant properties which provides both process related advantages as well as benefits in relation to the final formulation.
Feel free to contact us for further information about our binders and fillers and for special requests.
Alsiano Solutions.
High-performance MCC with special functionalities
CEOLUS™ KG, UF and PH MCC products facilitate formulations and contribute to production efficiency thanks to innovative particle morphology
With less impurities and high-quality consistency, the CEOLUS™ MCC products directly contribute to quality improvement of pharmaceutical and nutraceutical formulations. CEOLUS™ PH covers every standard grade with high quality and CEOLUS™ KG and CEOLUS™ UF provides additional advantages thanks to innovative particle morphology:
CEOLUS™ KG is a highly compactible MCC with rod-shaped particles which enable to develop poorly compactible and/or high dose formulations. Also, it helps to solve tableting issues such as insufficient hardness, sticking or any kind of lamination (capping incl.).
CEOLUS™ UF is a porous MCC with balance of compactibility and flowability. Those grades are dedicated to solving tableting issues such as insufficient API content uniformity or over-lubrication.
MCC with low nitrite/nitrate content
CEOLUS™ microcrystalline cellulose grades feature an exceptionally low concentration of nitrite and nitrate. Learn more >
Pharmacopoeia listings
JP: Microcrystalline Cellulose.
USP/NF: Microcrystalline Cellulose.
Ph. Eur.: CELLULOSE, MICROCRYSTALLINE.
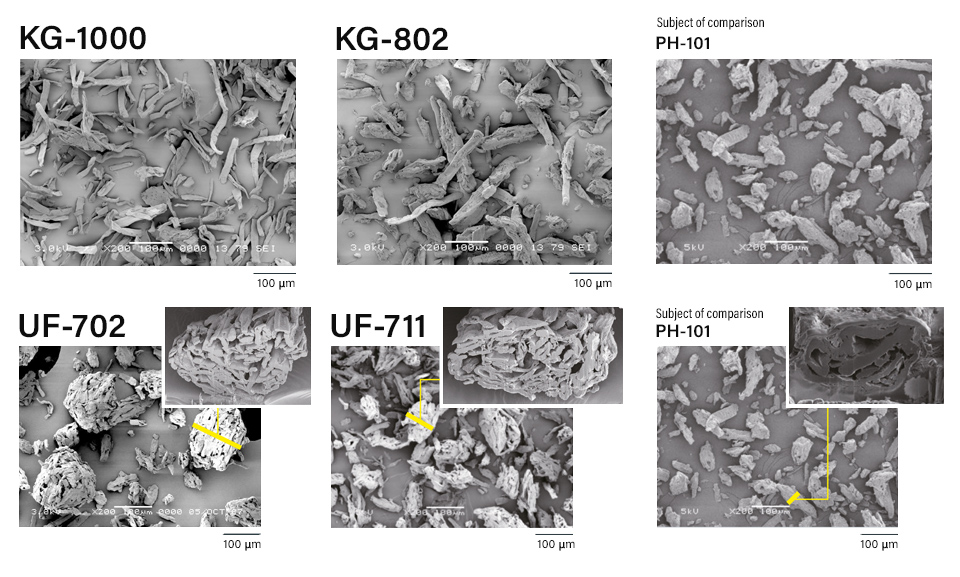
CEOLUS™ KG og UF grade lineup and comparison with CEOLUS™ PH-101 standard grade.
CELPHERE™ – a 100 % MCC sphere
European patented MCC seed core for pharma formulations
CELPHERE™ seed core is a special microcrystalline cellulose product (MCC) for solid dosage forms of pharmaceuticals. It has high sphericity, a narrow particle size distribution and high mechanical strength which facilitates precise dissolution profiles of controlled release formulation. In addition, it can improve yield due to reduced agglomeration and tolerance with high stress and coating machine varieties.
Other advantages of CELPHERE™ MCC include smooth surface, low friability, inertness, robustness and perfect flow.
CELPHERE™ MCC’s functional benefits make it an excellent excipient for formulation of patient-centric formulations and personalised medicine.
Pharmacopoeia listings
JPE: Microcrystalline Cellulose Spheres
USP/NF: Microcrystalline Cellulose
Ph. Eur.: CELLULOSE, MICROCRYSTALLINE
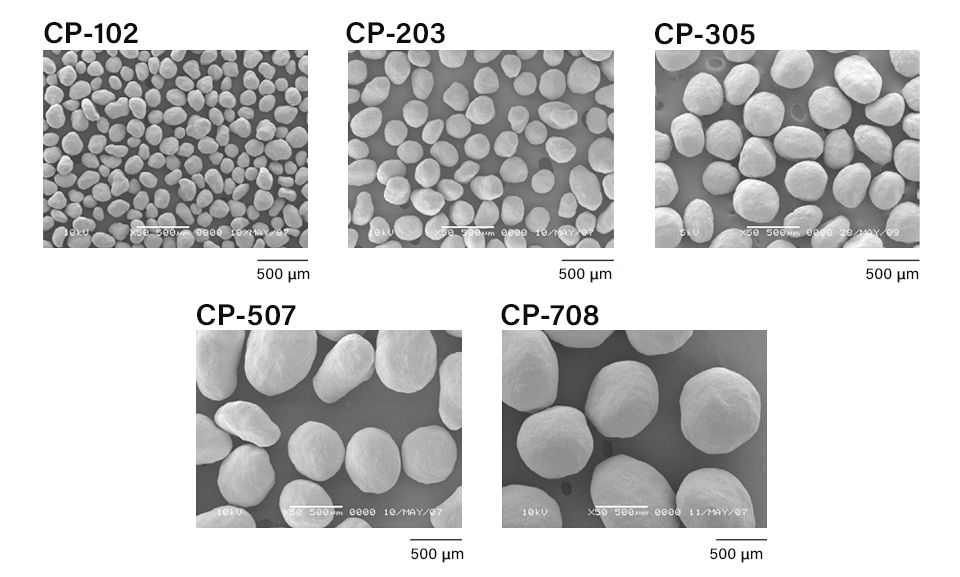
CELPHERE™ grade lineup
SEPISTAB™ ST 200 Phama
Binder and disintegrant based on partially pregelatinised corn starch
SEPISTAB™ ST 200 Pharma is a mixture of native starch and pregelatinized corn starch in free-flowing granular powder form. The pregelatinized starch provides both binding and disintegrating properties to formulations and the native starch improves the flow. These combined, makes it a premium highly versatile excipient perfect for direct compression and capsule filling.
Processing benefits
- SEPISTAB™ ST 200 PHARMA improves the flow of powder, an important feature for direct compression or filling capsules
- Due to its porosity SEPISTAB™ ST 200 PHARMA can be used as absorption medium
- SEPISTAB™ ST 200 PHARMA has an anti-capping action.
Formulation benefits
- SEPISTAB™ ST 200 PHARMA is a moisture scavenger with very low water activity, improving the stability of active ingredients sensitive to moisture.
- Use SEPISTAB™ ST 200 PHARMA to improve the hardness of the tablets without compromising their rapid disintegration in the body.
SEPISTAB™ ST 200 PHARMA complies with the European Pharmacopoeia pregelatinized corn starch monograph and is manufactured in accordance with IPEC GMP standards.
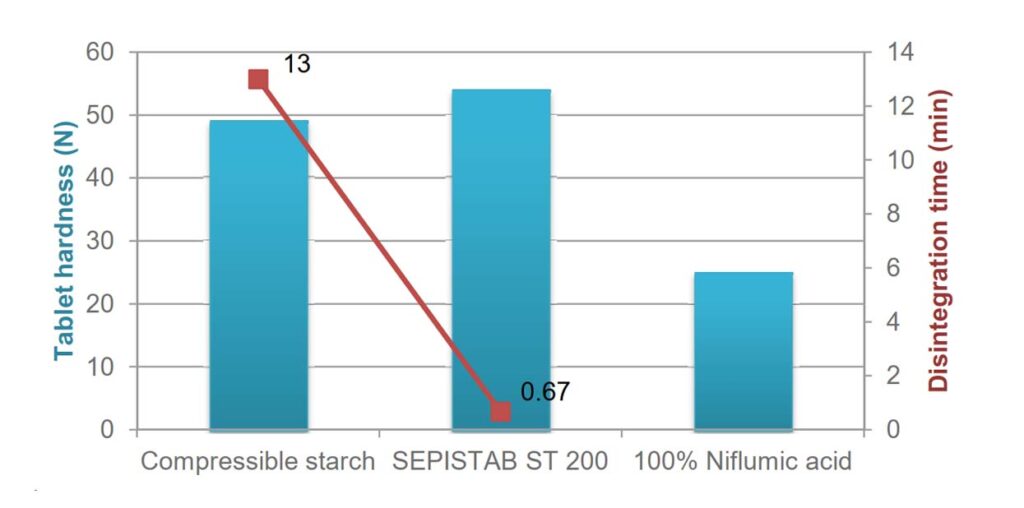
Figure: The performance of SEPISTAB™ ST 200 PHARMA as a binding and disintegrating agent.
Contact.
Let's talk ingredients for pharma applications
Lene Aarøe Nissen
Head of Nordic Sales
+45 22 70 10 02
lan@alsiano.com