Contributing to lower risk of potentially carcinogenic nitrosamine impurities in pharmaceuticals
To address the concern of potentially carcinogenic nitrosamine impurities in pharmaceuticals and nutritional supplements, Asahi Kasei introduces CEOLUS™ microcrystalline cellulose (MCC) with nitrite concentrations of 0.1 μg/g (ppm) or lower.
Following the detection of such impurities in pharmaceuticals in 2018, global awareness of the associated health risks has significantly heightened. In response, the pharmaceutical industry, guided by regional authorities like the European Medicines Agency (EMA) and the U.S. Food & Drug Administration (FDA), has conducted extensive assessments and research to pinpoint the root cause of these impurities.
One identified risk factor for the formation of nitrosamine is nitrosation, a reaction between secondary or tertiary amines with nitrites during or after the manufacturing process of drug substances and drug products. Reducing nitrite concentrations in pharmaceutical raw materials is considered an effective strategy to lower the risk of nitrosamine formation.
Minimising nitrite level in various CEOLUS™ grades
Asahi Kasei has been producing CEOLUS™ MCC in Nobeoka, Miyazaki, Japan, since 1970. MCC, derived from natural pulp, serves primarily as an excipient in pharmaceuticals, nutritional supplements, and foods. Notably, CEOLUS™ acts as a tablet binder, aiding in tablet formation and enhancing volume. High-performance CEOLUS™ grades in particular enable manufacturers to tackle formulation challenges, solve tableting issues, and create unique and patient-friendly dosage forms.
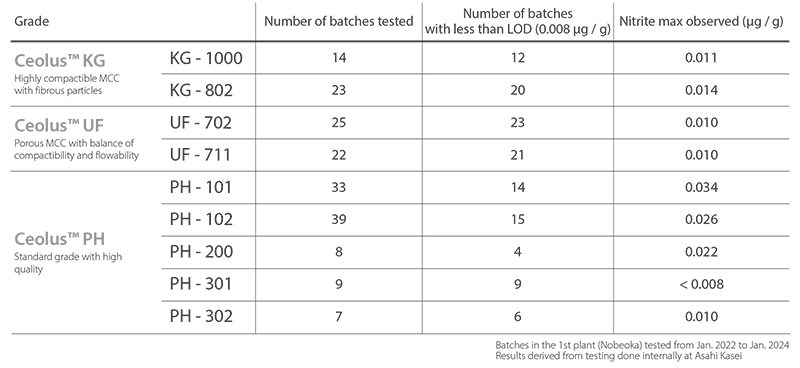
To reduce potential health hazards due to nitrosamine impurities, Asahi Kasei has succeeded in maintaining nitrite concentrations in CEOLUS™ at 0.1 ppm or lower.
Levels of nitrite content in CEOLUS™ MCC
Test results
Start of a second plant
In January 2024, Asahi Kasei commenced full commercial operations at its second CEOLUS™ manufacturing facility, located at its Mizushima Works in Okayama Prefecture, Japan. This expansion not only increases supply capacity but also enhances supply stability by enabling production at multiple sites.


